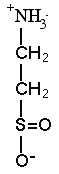PAUL H. YANCEY
Carl E. Peterson Endowed
Chair of Sciences Emeritus Professor
of Biology; Senior Research Scientist
Biology Dept., Whitman College,
Walla Walla WA 99362 USA
Curriculum Vitae
![]()
|
Click here for |
Click
here for |
VIDEO
online about our research: NHK
Documentary
DEEP OCEAN Pt 3 narrated by Sir Attenborough BIOGRAPHY: Click here for Curriculum Vitae
Prof. Yancey's hobbies include photography, woodworking, stained glass, hiking. |
|
Current/Recent Research--ORGANIC
OSMOLYTES: . |
||
|
PHYSIOLOGY |
|
My
Deep-Sea Biology website reformatted at Interview of me at The Reef Tank
|
|
RESEARCH: Prof. Yancey's research is described below, with additional information found by clicking the blue topics: |
|
OVERVIEW
More information can also be found by at Prof.
Yancey's SENIOR RESEARCH page.
|
IN THE NEWS:
 2018: --Our study on gel tissue in deep-sea fish including a robotic snailfish --NHK Documentary DEEP OCEAN Pt 3 narrated by Sir Attenborough --BBC's Blue Planet 2, Episode 2 |
TEAM OSMOLYTE
|
|
RECENT
and CURRENT OSMOLYTE RESEARCH:
See NEWS stories above
|
|
1.
DEEP-SEA FISHES, AMPHIPODS down to the Mariana Trench: High
Pressure Adaptations:
See
HADES - NSF grant and
NHK Documentary DEEP OCEAN Pt 3 narrated by Sir David Attenborough 2. MUSSELS: Intertidal stresses and osmolytes 3. MIGRATORY FISH: Salmon, European eels, sticklebacks; 6-gill sharks and Arctic skates 4. MAMMALIAN BRAIN CELLS: Dehydration & sports drinks with osmolytes |
|
Trimethylamine
oxide (TMAO) |
DETAILS: Prof. Yancey and others have found that some osmolytes, especially methylamine types such as TMAO (left), can actually stabilize proteins and counteract destabilizing effects of perturbants such as urea, salt, temperature and pressure. TMAO has a breakdown product, TMA (trimethylamine), that makes marine animals smell "fishy." Methylamines are high and appear to protect proteins in i) sharks and relatives, which also have the perturbing compound urea as an osmolyte; ii) mammalian (including human) kidneys, which must concentrate urea as a perturbing waste; iii) deep-sea animals which must cope with protein disturbances from high pressure. Our discovery of TMAO's role in the deep sea was featured in a New Scientist news story in 1999 and in a 2012 cover story of Science News. See Deep-sea Fish page for pictures and Deep-Sea Research page for research details. --Stabilizing properties of osmolytes may have practical applications, e.g., Welch and colleagues have shown that TMAO and other osmolytes can prevent the damaging protein of "mad-cow" disease from forming, and can cause the malformed protein of cystic fibrosis to fold properly. (Dr. Yancey assisted in one of the latter studies; see Howard et al. reference below in Research Area 2.) --We are also studying the role of osmolyte-type solutes in animals at hydrothermal vents and gas seeps, which have high levels of hydrogen sulfide, a gas toxic to most animals. A major osmolyte in shallow-water marine invertebrates such as clams and crabs is taurine. Taurine is also essential for mammalian brain development, and is the primary ingredient in many so-called energy or sports drinks (hint: the name taurine is derived from Taurus [bull]). Researchers in France have found high levels of the taurine derivatives hypotaurine and thiotaurine in clams, mussels and tubeworms which have sulfide-oxidizing bacterial symbionts. Thiotaurine, a product of hypotaurine and sulfide, may be a mechanism to prevent sulfide toxicity. We have found hypotaurine and thiotaurine in vent snails, limpets and heat-loving paralvinellid worms without symbionts, and shown that thiotaurine levels vary with sulfide exposure in these animals kept in laboratory pressure chambers. See Seeps and Vents page for pictures and Deep-Sea Research Page for research details. --Other researchers have found that the common osmolyte of marine algae, DMSP (dimethylsulfonoproprionate), breaks down into the gas DMS (dimethylsulfide), which is largely responsible for the "smell of the sea" that evokes emotional responses to the ocean. DMS is also thought to trigger the seeding of clouds, in what may be a global temperature negative feedback process. This is one of the postulates of the so-called Gaia hypothesis, which suggests that global warming will cause more DMS production, which via cloud formation may cool the planet. -- We have recently been working on DMSP and other osmolytes in coral animals and their symbionts, with Dr. Mary Hagedorn,who is hoping to cryopreserve coral larvae for potential re-seeding of decimated reef habitats. |
|
PUBLICATIONS: Click for a full list of Prof. Yancey's publications. Papers are listed below by theme/area |
|
I. REVIEW and COMMENTARY ARTICLES A. REVIEW ARTICLES on osmotic
balance and cytoprotection using osmolytes (RED = recommended
reading for overview on osmolytes)
|
| II.
MARINE / COMPARATIVE PHYSIOLOGY: ADAPTATIONS to SALINITY,
TEMPERATURE, EXERCISE, SULFIDE, PRESSURE
A. SHALLOW OCEANS:
SHARKS and relatives, BONY FISH; CORALS; MUSSELS (*undergraduate
co-authors):
|
|
|
B. DEEP-SEA ANIMALS -- PRESSURE; HYDROTHERMAL VENTS and COLD SEEPS (*undergraduate co-authors):
|
|
C.
MISC: RNA in DEVELOPMENT; MUSCLE physiology
|
III.
MAMMALIAN/MEDICAL RESEARCH: KIDNEY
and BRAIN OSMOLYTES
(undergraduate
co-authors*):
|
||
Normal kidney cells growing in tissue culture |
Kidney cells in culture exposed to 1mM Ibuprofen |
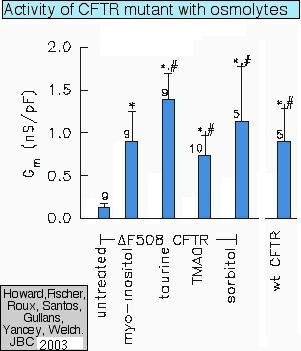 Restoring cystic-fibrosis channel function with osmolytes (see Howard et al., 2003) |
Back
to Research Index; Back
to top of page; GO TO Whitman
Biology Home Page










 .
. .
. .
.